L-Glutamate KIT YAMASA NEO
L-glutamate has been known as an “umami” ingredient. Its sodium salt, L-monosodium glutamate, has been used as the main ingredient of “umami seasoning.” It also functions as an excitatory neurotransmitter through glutamate receptors in animal. Therefore, detecting and quantifying L-glutamate is important in the fields of food and biochemistry.
For the measurement of L-glutamate, amino acid analyzers, glutamate dehydrogenase, and decarboxylase are employed. However, such methods lack accuracy and reproducibility, and pose problems, such as complicated procedures and high costs.
In 1983, during research on soy sauce, YAMASA CORPORATION discovered that some Streptomyces species produce glutamate oxidase, which specifically acts on L-glutamate, 1) and succeeded in its commercialization. Using this enzyme, a method that allows easy measurement of L-glutamate was also developed (see the measurement principles). 2),3) Based on this method, the YAMASA L-Glutamate Assay kit was developed, 4) which was improved in 2008 to launch the YAMASA L-Glutamate Assay Kit II.
To produce the present kit, the YAMASA L-Glutamate Assay Kit II was further improved. This kit includes ready-to-use measurement reagents (see the product contents), facilitating the measurement of L-glutamate (see the measuring method).
This kit is based on a chromogenic method for the determination of L-glutamate using L-glutamate oxidase which exclusively acts on L-glutamate. Ascorbic acid (vitamin C) is removed from samples by ascorbic acid oxidase in R1 enzyme reagent solution. Subsequently, L-glutamate oxidase in R2 enzyme reagent solution oxidize L-glutamate and produce hydrogen peroxide (1). Violet dye is produced from TOOS and 4-aminoantipyrine (4-AA) through hydrogen peroxide is oxidized by peroxidase. L-glutamate concentrations in the samples are calculated from the absorbance of violet dye (555 nm) (2).
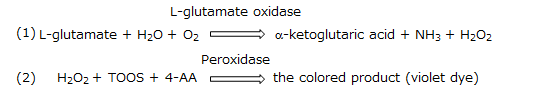
[Features]
This product has the following features, not observed for the conventional product (80057 YAMASA L-Glutamate Assay kit II), for the measurement of L-glutamate.
– This product includes ready-to-use solution reagents.
– Conventional enzyme reagents are dissolved in accompanying buffer solutions immediately before use. After dissolution, they are stable for one month. However, both enzyme reagent solutions in this product can be used until the expiration dates listed on the product labels.
– Only a small amount (only 10 mL) is required for measurement.
– The measurement range is wide (10-1,500 mg/L).
– Conventional pretreatment for samples containing ascorbic acid is not required even for samples containing ascorbic acid at 1,000 mg/L.
– The number of measurable samples, storage methods, and expiration dates are the same as those of the conventional product. In addition, the measured values are comparable with those of the conventional product.
[Product comparison table]
The present product is compared with the conventional product.
| Items for comparison | L-Glutamate Assay kit YAMASA NEO | YAMASA L-Glutamate Assay kit II | ||||
|---|---|---|---|---|---|---|
| Components | R1 enzyme reagent solution | 30mL | 1 vial | 50 mM Good’s buffer (pH 7.1) | 60mL | 1 vial |
| R2 enzyme reagent solution | 30mL | 1 vial | Enzyme reagent (lyophilized) | 1 vial | ||
| L-Glutamate Standard solution (250 mg/L) | 0.5mL | 1 vial | L-Glutamate Standard solution (100 mg/L) | 1.5mL | 1 vial | |
| Amount of sample | 0.01mL | 0.06mL | ||||
| Measurement range | 10~1500mg/L | 10~500mg/L | ||||
| Samples containing ascorbic acid | No pretreatment required (no effect up to 1,000 mg/L ascorbic acid) | Pretreatment required (about 30% decrease in measured values at 100 mg/L ascorbic acid) | ||||
| Number of tests | 66 | |||||
| Storage | 2~8℃ | |||||
| Shelf life | 12 months after the date of manufacture | |||||
[Comparison of measured values]
Various samples were measured using the present and conventional products. The measured values of this product were 94-106% of the conventional product.
| Samples | L-glutamate concentration (mg/L) | Present product/Conventional product | |
|---|---|---|---|
| Present product | Conventional product | ||
| Grace’s medium (10% FBS) | 700 | 747 | 94% |
| Tomato juice | 2,032 | 2,072 | 98% |
| Soy sauce | 10,701 | 10,850 | 99% |
| Commercial soup stock solution | 22,327 | 22,673 | 98% |
| Sausage solution | 200 | 189 | 106% |
(Notes)
– After extraction and removal of insoluble matters from solids, samples are appropriately diluted for measurement.
– The indicated concentrations are calculated from the measured values and the dilution rates.
– The above concentrations are our results, which do not reflect general L-glutamate concentrations.
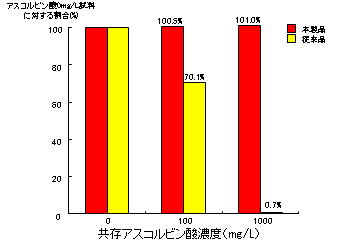
[Effects of ascorbic acid]
To prepare samples, ascorbic acid was added to L-glutamate solutions at 0, 100, and 1,000 mg/L. The samples were measured using the present and conventional products. Using the present product, no effects were observed up to 1,000 mg/L ascorbic acid.
– Product code: 80128
– This product includes:
R1 enzyme reagent solution 30 mL 1 bottle
R2 enzyme reagent solution 30 mL 1 bottle
L-Glutamate Standard solution (250 mg/L, lyophilized) 1 vial
*One kit allows measurement of about 66 samples (including standard solution, purified water, and sample dye to be measured simultaneously).
– Storage: 2-8°C in the dark place
– Shelf life: 12 months after the date of manufacture (for expiration date, see the label of the product package)
– Suggested retail price: ¥ 43,000
(1)Add 10 μL each of sample, standard solution, and purified water to tubes.
(2)Add 450 μL each of R1 enzyme reagent solution to the tubes, followed by mixing.
(3)Allow the tubes to stand at 20-30°C for 20 minutes.
For samples for which ascorbic acid needs not be removed, skip (3) and proceed to (4).
(4)Add 450 μL each of R2 enzyme reagent solution to the tubes, followed by mixing. Since the sample colors of dark samples may affect absorbance, prepare a test tube containing 10 μL of sample and 900 μL of purified water as a sample dye solution.
(5)Allow the tubes to stand at 20-30°C for 20 minutes, followed by absorbance measurement at 555 nm using purified water as a control.
| Test tubes for samples | Test tubes for standard solution | Test tubes for purified water | Test tubes for sample dye | |
|---|---|---|---|---|
| Samples | 10μL | – | – | 10μL |
| Standard solution | – | 10μL | – | – |
| Purified water | – | – | 10μL | 900μL |
| R1 enzyme reagent solution | 450μL | 450μL | 450μL | – |
| R2 enzyme reagent solution | 450μL | 450μL | 450μL | – |
| Absorbance | A | S | R | B |
<Calculation of concentrations>
The concentrations of L-glutamate in samples are calculated using the following formula:
L-glutamic acid(mg/L)=(A-B-R)÷(S-R)×250×dilution rate
– “Umami” ingredient analysis of food
L-glutamate contents in natto, tomatoes, soup stock, and soy sauce have been analyzed using this product.
– Measurement of glutaminase and peptidase activities
The activities of glutaminase, which converts glutamine into glutamate, and peptidase, which degrades peptides into amino acids, can be determined by measuring L-glutamate production. 5)
*Notes
The above examples of usage are based on measurement using L-glutamate oxidase or the conventional product.
– Dilute liquid foods, such as soy sauce, with purified water to adjust L-glutamate concentrations to 10-1,500 mg/L (e.g., 100-200-fold dilution for soy sauce).
– Cut solid foods, such as cheese and sausage, into pieces. Mix these pieces with 10-20 times the amount of purified water or phosphate buffer. Cool and filter the mixture. Dilute the filtrates 2-5 fold with purified water for sample preparation. For turbid samples, repeat filtration and centrifugation. Protein removal is not needed.
– Medium can be measured without dilution. Samples with concentrations above the measurement range need to be diluted with purified water.
1. Kusakabe H. et al. (1983). Agric. Biol. Chem. 47(6). 1323.
2. Yamauchi H. et al. (1987). J. Japan Soy Sauce Research Institute. 13(1). 8.
3. Kusakabe H. et al. (1984). Agric. Biol. Chem. 48(1). 181
4. Maruyama H. et al. (1989). J. Japan Soy Sauce Research Institute. 15(6). 239.
5. Kusakabe H. et al. (1984). Agric. Biol. Chem. 48(5). 1357.
